Select Your Region
Medical Silicone Compliance: Why It Matters

Healthcare-grade silicone elastomers must meet strict standards for purity, clarity, durability, and biocompatibility. Momentive’s Silopren™ LSR 4000 series is engineered to comply with these requirements, making it ideal for both disposable and reusable medical devices.
Key Compliance Features

- Biocompatibility: Safe for contact with body fluids and medications
- Regulatory Standards: Meets stringent healthcare regulations and ISO, USP Class VI compliance, and FDA requirements
- Sterilization Resistance: Maintains performance after repeated autoclave, gamma, or EO sterilization
- Primerless Adhesion: Bonds to substrates like PC, PBT, and copolyesters without post-curing
- Aging and Chemical Stability: Withstands harsh environments and extreme temperatures
Medical Silicone Specification Checklist
When selecting silicone for healthcare applications, ensure your material meets the critical specifications below.Requirement
|
Silopren™ LSR 4000 Series Advantage
|
|---|---|
Biocompaibility
|
Compliant with ISO, Class VI, FDA standards
|
Sterilization Durability
|
Stable after multiple sterilization (autoclave, gamma, or EO) cycles
|
Transparency & Clarity
|
High optical clarity for diagnostic devices
|
Durometer Range
|
Wide Shore A hardness (~30A) options
|
Fast Cure & Tear Strength
|
Supports high-speed injection molding processes
|
Self-Bonding Capability
|
Primerless adhesion to plastics and metals
|
Design Flexibility
|
Enables soft-hard overmolding and insert molding
|
- General Medical Parts: O-rings, gaskets, seals, valves, diaphragms
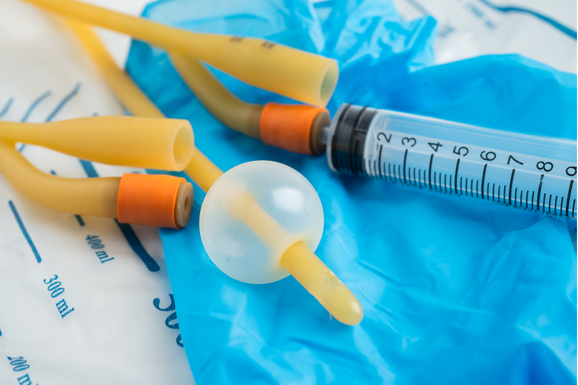
- Drug Delivery Systems: Catheters, tubing, dosage control devices
- Respiratory & Surgical: Masks, valves, wound drains
- Dental & Orthopedic: Impression molds, prosthetic components
Scan. Connect. Solve.
Need help right now?
Talk to a Technical Specialist in Seconds
Simply scan the QR code with your smartphone to start a conversation with one of our technical specialists for Momentive silicone elastomers.
Talk to a Technical Specialist in Seconds
Simply scan the QR code with your smartphone to start a conversation with one of our technical specialists for Momentive silicone elastomers.


Thank you
Thank you for your inquiry and interest in ChemPoint.
We will respond to you shortly.
ChemPoint will not under any circumstances release personal user information to individuals or companies. All information collection is solely used to support ChemPoint customers service communications. Read our Privacy Notice.
Are you in the correct region?
We’ve detected that you are located in a different region than the region selected on the website. Would you like to change your region?
Current Region: English - United States