Select Your Region
Vazo™ Free Radical Initiators

Today, significant portions of consumer goods, electronics, vehicles used for transportation, and the built environment are made using plastics or elastomers composed of vinyl polymers. These polymers, such as PE, PP, PVC, PS, PVAc, and PAN, are typically produced by free radical polymerization involving controlled reactions using vinyl monomers, radical initiators, chain transfer agents, and a solvent.
One reason vinyl polymers are so prevalent is because of the high degree of durability and versatility they can provide. Achieving these characteristics, however, often depends on the quality of the polymer, which is dictated by a careful manufacturing process that optimizes molecular weight, purity, and morphology. In many cases, these properties can be optimized and controlled quite easily if azonitrile-based radical initiators, or azo initiators, are used.
Azo initiators are organic compounds with a central N2 linking group, as shown in the chemical decomposition scheme below. These compounds are typically used as thermal initiators and are incredibly useful as a free radical source for polymerization initiation because it is thermodynamically favorable for the azonitrile compound to drive the release of N2 molecules as nitrogen gas.
What Are Azo Initiators?
Azo initiators are organic compounds with a central N2 linking group, as shown in the chemical decomposition scheme below. These compounds are typically used as thermal initiators and are incredibly useful as a free radical source for polymerization initiation because it is thermodynamically favorable for the azonitrile compound to drive the release of N2 molecules as nitrogen gas.

The formation of two radicals from the azonitrile compound is key in this reaction. These radicals initiate polymerization by attacking the double bond of vinyl monomer molecules, forming a new radical species that repeats this process with other vinyl monomers, ultimately propagating the polymer chain.
Benefits of Using an Azonitrile Initiator
One of the more commonly used initiators for radical initiation is organic peroxide because it is relatively inexpensive. However, it has disadvantages for maintaining polymer quality, workplace safety, and simplicity of use. Therefore, many vinyl polymer manufacturers invest in azo initiators to gain better polymer yields, spend less on hazard response and mitigation programs, and increase their manufacturing efficiency. Details pertaining to how azo initiators specifically provide these benefits in comparison to organic peroxides are explored below.
Optimized Polymer Quality |
||
| Organic peroxide initiators decompose into hydroxyl radicals, which are extremely strong oxidizers. These hydroxyl radicals can easily abstract hydrogen atoms from growing polymer chains, creating radicals at unwanted sites. When this occurs, unexpected polymer branching or cross-linking may occur, presenting significant challenges to controlling the molecular weight and morphology of the polymer. On the other hand, azonitrile radical initiators do not abstract hydrogen from growing polymer chains. This reduces the chances of undesired polymer branching and cross-linking, giving the manufacturer more control over the final polymer structure. Therefore, azonitrile radical initiators enable crucial quality improvements for manufacturers who produce specialty polymers with well-defined molecular weight and quality goals. |  |
|
Increased Safety |
||
| Since organic peroxides are incredibly volatile initiators, they can undergo a rapid exothermic reaction through decomposition if they are not stored under strictly controlled temperatures. This, of course, can lead to explosion risks that pose serious workplace hazards. Compared to peroxides with similar half-lives, azonitrile radical initiators have higher decomposition temperatures, making them less likely to decompose accidentally and endanger workplaces. Also, azonitriles are not shock sensitive like organic peroxides. Therefore, the risk of explosion during transportation and storage is reduced. In addition, azonitriles pose lower health risks while handling because they are characterized by low toxicity and are not skin sensitizers. |  |
|
Simplified Reaction Conditions |
||
| Due to their extremely high reactivity, organic peroxides present challenges in setting up reaction conditions that maximize polymer yield and quality. For example, peroxides may react with redox-sensitive agents, solvents, and dyes in a reaction medium, making it difficult to adjust reaction condition requirements to promote effective use. In comparison, azonitriles will not undergo redox reactions or be compromised by redox-sensitive reagents. Azo radical initiators also will not react with solvents, dyes, or pigments. In addition, organic peroxides tend to undergo radical-induced decomposition, cannibalizing other initiators and making polymerization less efficient. Comparatively, azonitriles do not decompose in the presence of free radicals—rather, only in the presence of heat or light. This allows them to be used with other types of radical initiators if desired. |  |
Vazo™ Free Radical Initiators
Vazo™ initiators are substituted azonitrile compounds manufactured in the United States by Chemours. These free radical initiators are ideal for initiating polymerization and creating polymers with strictly defined molecular weights and morphologies. Vazo™ is available in six grades that vary by decomposition temperature and solubility in either water or organic solvents.Selecting a Vazo™ Free Radical Initiator
The right Vazo™ grade for an application is decided by two main factors: the desired reaction temperature and the reaction medium.
Reaction Temperature
The most important factor in determining which Vazo™ Free Radical Initiator to use is the reaction temperature, typically determined by the specific monomer used in the reaction. In general, the Vazo™ portfolio spans a realistic operating window between 65°C and 140°C. The number in each Vazo™ grade denotes the approximate 10-hour half-life temperature. As a guideline, a suitable Vazo™ grade may be selected based on a reaction temperature range that is 10°C to 30°C above or below the corresponding grade number.
Reaction Medium
The solubility of the initiator in the desired reaction medium is critical to polymerization efficiency. All Vazo™ grades are soluble in common reaction mediums such as toluene and acetone. However, Vazo™ 67 exhibits better solubility in a broader range of organic solvents. For reactions in which water is present, Vazo™ 56 WSP and Vazo™ 68 WSP are recommended.
| Grade | 10-Hour Half-Life Temp (°C)
|
Benefit
|
Molecular Structure
|
|---|---|---|---|
52
|
Great in low-temperature polymerizations
|
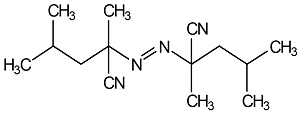 |
|
64
|
Cost-effective
|
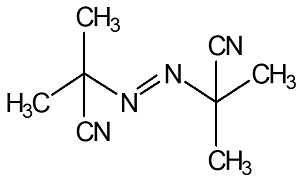 |
|
|
|
67
|
Best solubility
|
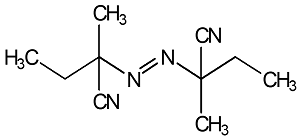 |
88
|
Ideal for high-temperature polymerizations
|
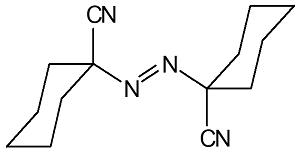 |
|
Vazo™ 56 WSP
2,2’-azobis-(2-amidinopropane) dihydrochloride 2,2’-azobis(2-methylpropionamidine) dihydrochloride AAPH |
56
|
Water-soluble grade
|
 |
Vazo™ 68 WSP
4,4′-azobis-(4-cyanovaleric acid) 4,4′-azobis-(4-cyanopentanoic acid) ACVA |
68
|
Soluble in water and organic solvents
|
 |
Applications for Vazo™ Free Radical Initiators
Examples of vinyl polymers ideal for synthesis using Vazo™ free radical initiators include PE, PMMA, PS, PVC, PVAc, and PAN. As alluded to at the beginning of this article, these polymers are prevalent in various components and structures. Below are some of the use cases for these polymers and the benefits Vazo™ initiators provide that increase performance.
| Industry | Product | Polymer | Vazo™ Benefits |
| Transportation | Tires | Styrene-butadiene rubber (SBR) | Minimal chain defects |
| Hoses | PE, PVC, SBR | ||
| Clear Coatings | Acrylic | Minimal chain defects Minimal color-forming impurities | |
| Paints | Acrylic | ||
| Safety Glass | Acrylic | ||
| Carbon Fiber | PAN | Minimal chain defects Permits higher molecular weights No oxygen-containing groups |
|
| Water Treatment | Flocculants | Polydiallyldimethyl ammonium chloride (PolyDADMAC) | Minimal chain defects |
| Polyacrylamide | |||
| Treatment Pond Liners | PE, PVC, PP, EPDM | ||
| Medical and Personal Care | Medical Tapes | Acrylic | Minimal impurity formation Minimal chain defects FDA approval in food contact applications |
| Hair Spray Binders | PVP, PVA | ||
| Binders for Aspirin Coatings | PVP, PVA | ||
| Electronics | Computer Housings | PS | Minimal chain defects No oxygen-containing groups |
| Electronic Circuits | PMMA, Polyethylene naphthalate | ||
| Copper Laminates | PE, PTFE | ||
| Photoresist Coatings | PMMA, Cyanoacrylates | ||
| LCD Backlight Guide Plate | PMMA | ||
| Paper and Textiles | Coatings | LDPE, PET, PTFE | Minimal chain defects Minimal color-forming impurities FDA approval in food contact |
| Dyes | Acrylates | No oxygen-containing groups | |
| Release Agents | PTFE, PE | Minimal chain defects | |
| Acrylic Fibers | Acrylic | Minimal chain defects |
Summary
Manufacturers of vinyl polymers and resins often use organic peroxide initiators for free radical polymerization because they are relatively low in cost. However, organic peroxides contribute to issues with polymer quality, workplace safety, and manufacturing efficiency. Vazo™ azonitrile-based radical initiators, manufactured in the United States, provide manufacturers with a better initiator alternative that creates polymers with fewer chain defects and tighter molecular weight specifications. Vazo™ initiators are also less susceptible to accidental degradation and require fewer reaction conditions for effective use. Thus, they permit higher efficiency and are much safer and easier to use than organic peroxide initiators.
Do you have any questions about Vazo™ or need help selecting the right grade? Click below to have one of our product specialists guide you to the right Vazo™ radical initiator solution for your application.

Thank you
Thank you for your inquiry and interest in ChemPoint.
We will respond to you shortly.
ChemPoint will not under any circumstances release personal user information to individuals or companies. All information collection is solely used to support ChemPoint customers service communications. Read our Privacy Notice.
Are you in the correct region?
We’ve detected that you are located in a different region than the region selected on the website. Would you like to change your region?
Current Region: English - United States